Poliomyelitis is a highly contagious disease caused by a virus that attacks the nervous system. Last week, the **contamination of the oral polio vaccine** that was reported when the Health ministry when it found a batch of oral polio vaccines (made by Biomed pharmaceuticals in Ghaziabad), was contaminated with poliovirus type 2. This is just one among the many OPV manufacturers in India, and it puts 1 in 200,000 children who have been administered the OPV at risk. This should not set alarm bells ringing. But before we get to the issue, here’s a quick primer on the landscape of polio vaccines and the disease itself. Poliomyelitis Children younger than 5-years are more likely to contract the polio virus. According to the World Health Organisation (WHO), 1 in 200 polio infections will result in permanent paralysis. 1 in 200 infections leads to irreversible paralysis. Among those paralysed, 5 to 10 percent die when their breathing muscles are affected. However, thanks to the Global Polio Eradication Initiative (GPEI) in 1988, the world is almost polio-free: except for countries where polio is still present – Pakistan, Afghanistan and Nigeria. Poliomyelitis is caused by an infection with an entero-virus known as poliovirus (PV), which colonises the gastrointestinal tract. The incubation period (to the first signs and symptoms) ranges from three to 35 days, with a span of six to 20 days. PV infects and causes disease in humans alone. Three serotypes of poliovirus have been identified—poliovirus type 1 (PV1), type 2 (PV2), and type 3 (PV3). All three are extremely virulent and produce the same disease symptoms. PV1 is the most commonly encountered form, and the one most closely associated with paralysis. India is the latest country to have officially stopped endemic transmission of polio, with its last reported case in 2011. The World Health Organisation **formally declared India polio-free** on Thursday, 27 March, 2014, with no new case of the disease detected in the country in the past four years. There is no cure for polio, it can only be prevented. Polio vaccine, given multiple times, can protect a child for life. Oral polio vaccine (OPV) is used for prevention. CDC recommends that children get four to six doses of polio vaccine. [caption id=“attachment_5337791” align=“alignnone” width=“1280”]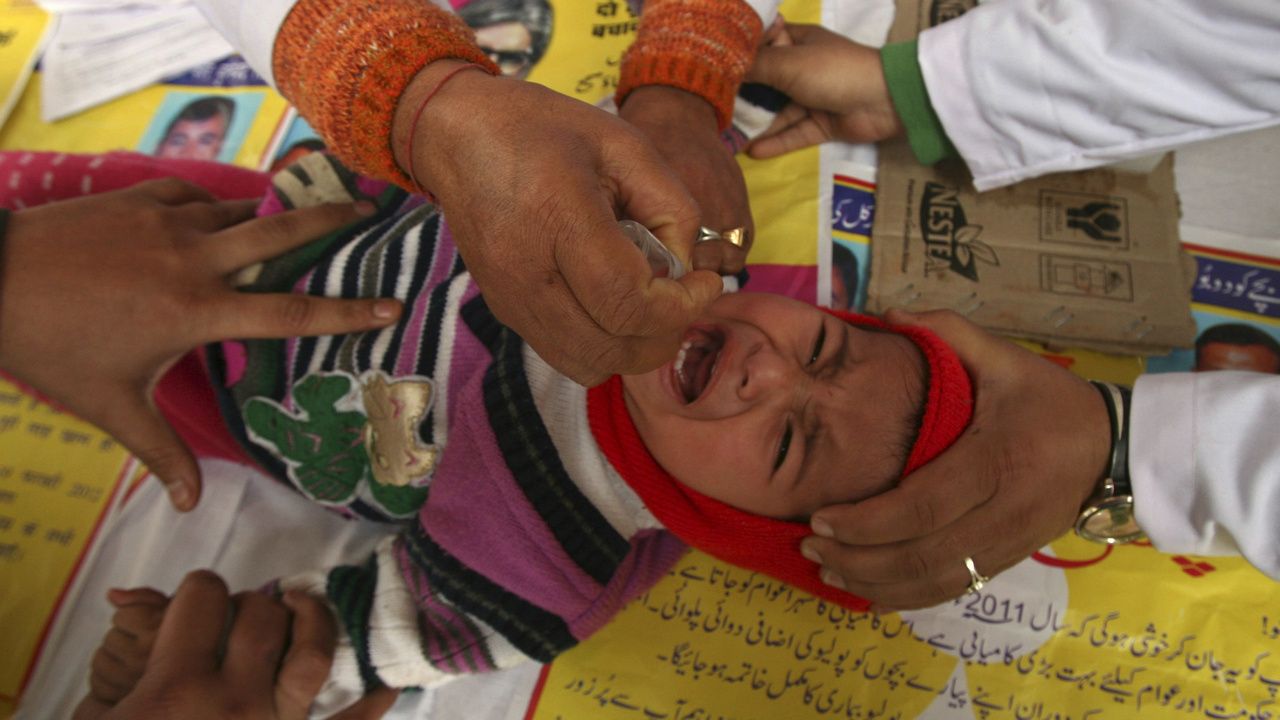 A child receives polio drops during a polio eradication programme in Jammu. File photo Reuters[/caption] IPV is Inactivated (killed) virus vaccine or you call it Injectable Polio Vaccine, whose weak point is that it does not provide local gut immunity. On the other hand, Oral Polio Vaccine (OPV) is a live attenuated vaccine, to be given by mouth; less immunogenic and therefore more doses are required (minimum 6). OPV consists of a mixture of live attenuated poliovirus strains of each of the three serotypes, selected by their ability to mimic the immune response following infection with wild polioviruses, but with a significantly reduced incidence of spreading to the central nervous system. The action of oral polio vaccine (OPV) is two-pronged. OPV produces antibodies in the blood (‘humoral’ or serum immunity) to all three types of poliovirus, and in the event of infection, this protects the individual against polio paralysis by preventing the spread of poliovirus to the nervous system. In very rare cases, the administration of OPV results in vaccine-associated paralysis associated with a reversion of the vaccine strains to the more neurovirulent profile of wild poliovirus. In a few instances, such vaccine strains have become both neurovirulent and transmissible and have resulted in infectious poliomyelitis. The Biomed OPV contamination There are multiple vaccine manufacturers in India along with the globe – and one company “BioMed” manufacturing OPV under the government licence from Ghaziabad in India had OPV containing type 2 viruses in them. So this doesn’t cause any harm to anyone except a theoretical possibility of vaccine-induced poliomyelitis in 1:200,000 population. Having said that, the vaccine was manufactured only by BioMed and a few batches were affected. Those batches were apparently sold in states of UP, Maharashtra and Telangana as per the information available. So, in essence, no one in Karnataka, Tamil Nadu, Kerala or any other state where this batch of vaccine was NOT used are at any risk at all. OPV (Oral Polio Vaccine) normally contained 3 viruses till 2016. Since polio caused by type 2 virus has been eliminated from the world since 1999, outbreaks of polio in most countries were due to either poliovirus type 1 or 3. WHO decided that the ‘risk’ of vaccine (because it is a live virus) induced poliomyelitis by type 2 poliovirus which is 1:200,000 is not acceptable anymore as the type 2 virus had not been found for over 2 decades. So they suggested that OPV should give multiple doses without any worry, but it could contain only type 1 and type 3 viruses — in that way leaving no scope for vaccine-derived poliomyelitis by type 2 poliovirus. But BioMed-manufactured OPV was found to be still manufacturing the OPV with all 3 viruses. Up to 1.5 lakh vials were manufactured by the company, of which two-thirds were administered to children. One-third of the batch has been withdrawn. This doesn’t mean it is dangerous, it means it is against WHO verdict and it unnecessarily puts 1:200,000 children who have received this vaccine to ‘contract’ vaccine-derived poliomyelitis from polio 2 virus. This information, if disseminated in the right way, is important otherwise it could damage the pulse polio programme being carried out by Government of India — which in turn can put India at risk of “importing poliovirus” from neighbouring Pakistan and Afghanistan. Having said that, India is still “Polio Free” and there are no wild cases of polio reported. The press could have been more responsible in putting out correct information rather than causing panic with incomplete information. I hope this article allays the fear of many common men and women with proper information. The author is the chairman and practising Neonatologist at Cloudnine Group of Hospitals
WHO decided that the ‘risk’ of vaccine-induced poliomyelitis by type 2 poliovirus is not acceptable as it had not been found for over 2 decades.
Advertisement
End of Article


)
)
)
)
)
)
)
)
)



