Researchers have developed a novel, low-cost finger prick blood test that deploys gold-plated nanoparticles for early detection of
cancer
. The research team from the University of New South Wales in Sydney used
nanoparticles
to latch on to the targeted
microRNAs
(miRNAs), even in ultralow levels, which enabled them to be easily extracted. “We are detecting small molecules found in the blood which could also identify the type of
cancer
, while they are looking for
rare cells
that are responsible for the spread of cancer,” said Justin Gooding, Professor from the University. In the study,
published in
the journal Nature Nanotechnology, the team reported modifying gold-coated
magnetic nanoparticles
with
DNA
to match the miRNA they wanted to detect. Gooding said the nanoparticles are, in effect, dispersible electrodes. [caption id=“attachment_5085231” align=“alignnone” width=“1280”]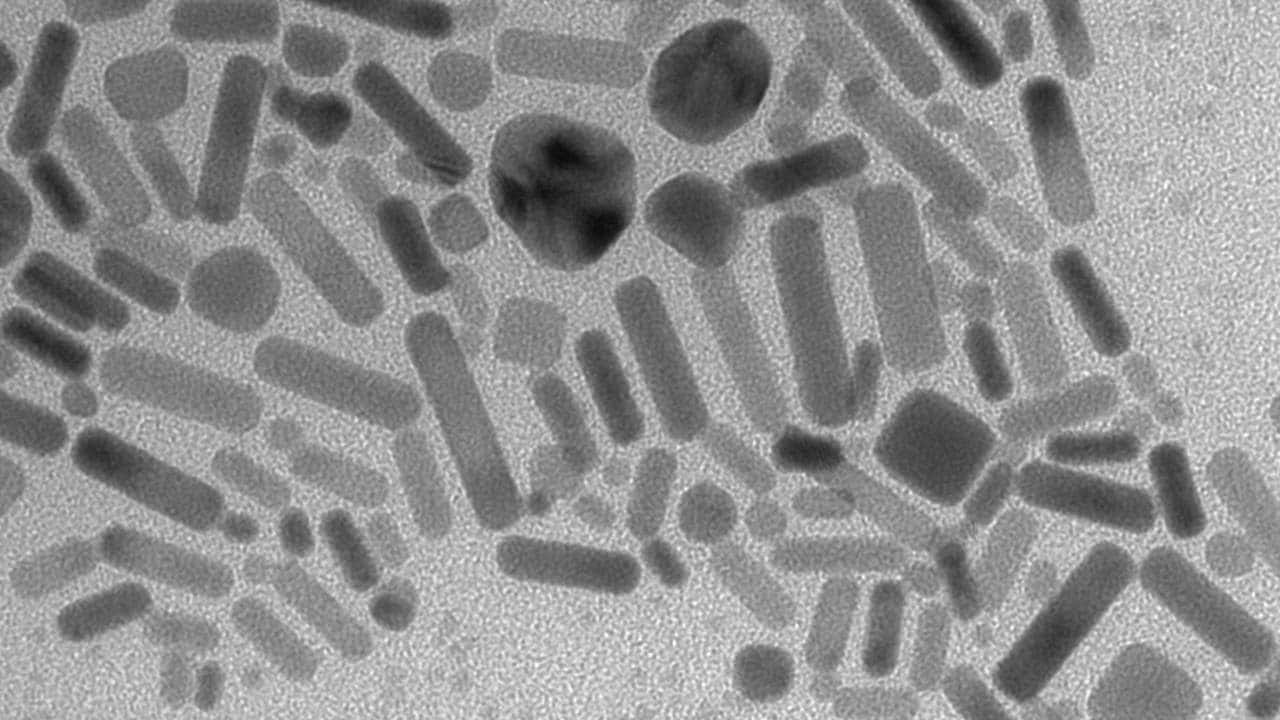 Gold nanoparticles deposited on the carbon substrate. Wikimedia Commons/Волочаев[/caption] When circulated through the blood they capture the miRNA before a magnet is used to recapture the
nanoparticles
with the newly attached microRNA. “Now we get more of the
microRNA
because the dispersible electrodes capture nearly everything in the sample,” Professor Gooding said. “Because the capture is so effective, we get higher sensitivities and can detect much lower limits. “And since we bring them back to the electrode under a magnet, our response time is much faster,” he noted. Moreover, the new
diagnostic technique
costs less and is faster than
traditional methods
. “Our method takes 30 minutes compared with almost 12 hours for quantitative polymerase chain reaction,” Gooding said. Gooding said he expects the technology to be available within three years, pending regulatory approvals.
Gold nanoparticles deposited on the carbon substrate. Wikimedia Commons/Волочаев[/caption] When circulated through the blood they capture the miRNA before a magnet is used to recapture the
nanoparticles
with the newly attached microRNA. “Now we get more of the
microRNA
because the dispersible electrodes capture nearly everything in the sample,” Professor Gooding said. “Because the capture is so effective, we get higher sensitivities and can detect much lower limits. “And since we bring them back to the electrode under a magnet, our response time is much faster,” he noted. Moreover, the new
diagnostic technique
costs less and is faster than
traditional methods
. “Our method takes 30 minutes compared with almost 12 hours for quantitative polymerase chain reaction,” Gooding said. Gooding said he expects the technology to be available within three years, pending regulatory approvals.
A new blood test that uses gold-plated nanoparticles for early cancer detection
Indo Asian News Service
• August 31, 2018, 11:00:01 IST
It is sensitive enough to detect lower limits of cancer than previously possible, says researcher
Advertisement
)
End of Article