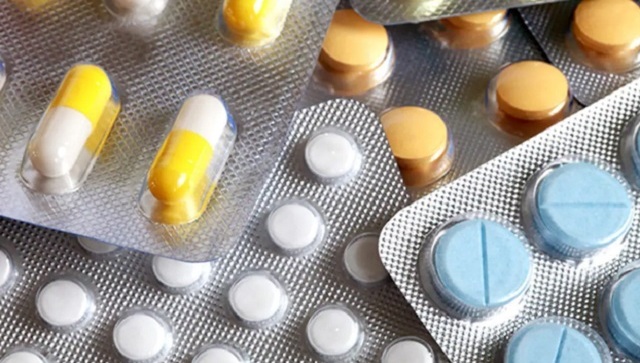
After nearly seven years of status quo, more than 140 consultation meetings with experts, and extensive studies, on 13 September, this year, the Union Ministry of Health and Family Welfare launched the revised National List of Essential Medicines (NLEM) 2022. NLEM was a regulatory concept that was initiated in 1996 to maintain a check on the pricing of critical drugs, and to ensure accessibility to affordable and quality medicines at all levels of healthcare. Out-of-pocket expenses in public health have been a persistent challenge for India, and combined with our country’s socio-economic profile, ensuring the affordability of critical drugs has consistently been critical in policy frameworks. It was with this perspective that the first National List of Essential Medicines was prepared in 1996, and since then it has been subsequently revised four times in 2003, 2011, 2015, and with 2022 revision being the latest. A reflection of the country’s evolving public health profile The NLEM has always been known to reflect and align with the evolving public health priorities of the country, and the current revisions made to the NLEM are a reiteration of the same. In the NLEM 2022, there has been an addition of 34 drugs and a deletion of 26 drugs, making the total approved list to 384 which come under 27 therapeutic categories. An important addition in NLEM 2022 has been of four anti-cancer medicines which support treatments for certain types of blood and lymph node cancers, colorectal and pancreatic cancers, etc. The addition of anti-cancer medicines in particular assumes importance, considering that India has one of the highest incidences of cancer cases globally (partially on account of a large population base), accounting for nearly 7 per cent of global cases. The country expects a further 11 per cent rise in cancer cases by the year 2025, according to ICMR studies. Therefore, the addition of anti-cancer medicines in the NLEM will play a crucial role in enhancing accessibility of often expensive oncology therapies, further making a positive impact on public health out of pocket expenses. There have been a number of other notable additions to NLEM, including cardiovascular medicines, the rotavirus vaccine, which is part of the government’s immunisation programme, and deletions like Rantidine, Sucralfate, white Petrolatum, Atenolol and Methyldopa, basis cost-effectiveness and availability of better drugs. The revision of the NLEM is an extremely thorough and exhaustive process, with set parameters for additions and deletions. Once revised, the Department of Pharmaceuticals, under the Ministry of Chemicals and Fertilizers adds them in the Drug Price Control Order (DPCO). The price is regulated by the National Pharmaceutical Pricing Authority (formed under department of Pharmaceuticals) which will enforce the provisions of DPCO. The prices of medicines under the NLEM cannot be increased by the companies themselves, but every year the prices are increased or decreased as per the Wholesale Price Index. Considerations for future revisions of the NLEM As this revision to the NLEM after seven years brings to the fore discussions on the various nuances around preparation and implementation of the list, certain perspectives may be considered for prioritising future revisions. One key aspect for consideration can be aligning the revision of the NLEM to a prescribed timeline. So far, the NLEM in its previous revisions has been updated in varying gaps of four to eight years, with a seven-year gap between the current and previous revision. In 2015, when NLEM was revised, the core committee on revision on NLEM had recommended that revisions should be made to the list every three years. This will ensure that the NLEM keeps an active watch on the evolving prevalence of diseases and is able to “preserve its relevance”. A second area for consideration should be the impact of the inclusion of medicines in the NLEM on the finances and R&D realisation of the pharmaceutical sector. Experts have pointed that price controls on drugs are a pro-public health concept prevalent across countries, like in India. However, there is a difference in India’s approach to drug-price control, in comparison to other countries like Italy, US, US, UK, Japan, Canada, etc. In the countries mentioned, the governments have adopted a reimbursement approach wherein the respective governments directly procure the drugs from pharmaceutical companies at fair prices and subsidise the final cost to the end consumers. In India’s case, however, the impact of the reduced price is not in-principle reimbursed to the pharmaceutical companies, which in turn impacts the realization of their massive expenditure on R&D, which the pharmaceutical sector is well-known for (pharmaceutical sector makes one of the highest expenditures on R&D owing to the long-timelines and complexities of drug-development processes). Therefore, to ensure that global and domestic pharmaceutical companies continue to feel incentivized for their R&D expenditure on highly prevalent and critical diseases, the Government should also explore means to rationalise their spend once their treatments get covered under the NLEM. In a nutshell, the concept of NLEM is extremely critical to the realisation of domestic and global-public health goals. However, certain perspective should be actively considered to ensure the sustainability and wider stakeholder acceptability of the exercise. The author is co-founder and CEO, Primus Partners. Views are personal. Read all the Latest News , Trending News , Cricket News , Bollywood News , India News and Entertainment News here. Follow us on Facebook, Twitter and Instagram.
The NLEM has always been known to reflect and align with the evolving public health priorities of the country, and the current revisions made to the NLEM are a reiteration of the same
Advertisement
End of Article


)

)
)
)
)
)
)
)
)



