India has launched a digital tracking system to monitor the movement, production and ingredients used in cough syrups as the government cracks down on contamination of the medicines, following the deaths of children in Madhya Pradesh.
According to a report by News18, the Central Drugs Standard Control Organisation (CDSCO) has directed regulators and manufacturers to register in the Online National Drug Licensing System (ONDLS) portal for real-time monitoring of solvent batches.
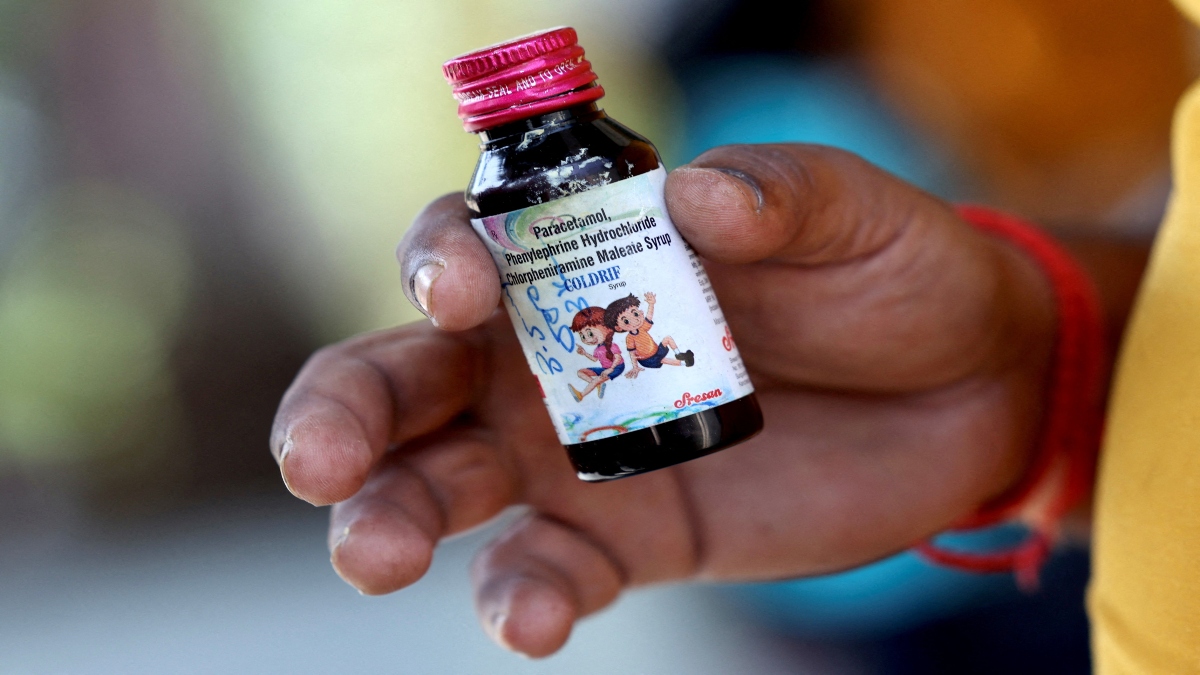
The move comes after the World Health Organisation said India has more work to do in halting sales of toxic cough syrup, despite some progress. The children died after taking the Coldrif cough medicine made by Sresan Pharma, which tests showed contained the toxin diethylene glycol in quantities nearly 500 times the permissible limit.
What are the new measures?
A CDSCO document has identified 10 solvents classified as high-risk, including glycerin, propylene glycol, sorbitol, maltitol, and ethyl alcohol, ingredients commonly used in syrups and other liquid medications. The new digital system aims to formalise oversight and address gaps in the monitoring of these raw materials, the document said.
“It has been decided that a Digital Monitoring System on the ONDLS portal needs to be established for monitoring the supply chain as well as quality of the high-risk solvents including the propylene glycol. Accordingly, the ONDLS portal has been upgraded and made live by this directorate for addressing this issue," CDSCO said.
Under the new system, all pharmaceutical manufacturers are required to obtain manufacturing licences through the ONDLS portal and submit real-time information about every batch that is produced.
Each batch record must provide details, including the batch number, quantity, certificate of analysis (CoA), and information about the buyer or vendor receiving the solvent. State regulators have been instructed to ensure that no batch is released to the market until all required data has been digitally uploaded and verified on the portal.


)
)
)
)
)
)
)
)
)



