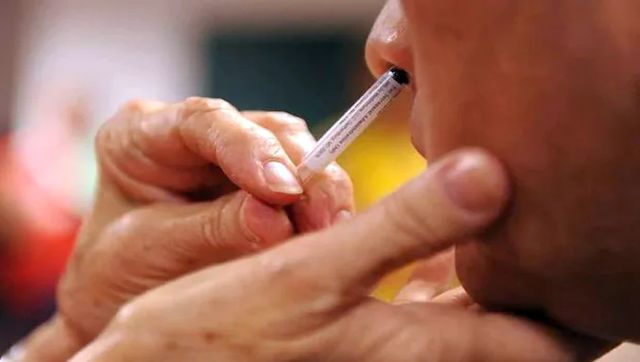
As China is witnessing a massive COVID surge, with patients thronging hospitals and stocks of medicine running out, India is also ramping up its fight against the virus. A day after Prime Minister Narendra Modi urged citizens to mask up and stay vigilant, the Union health ministry approved Bharat Biotech’s intranasal COVID vaccine. The Centre has announced that the two-drop vaccine, iNCOVACC, will be used as a booster dose for those aged 18 years and above and is likely to be introduced on the Co-WIN platform Friday evening. A source was quoted as telling news agency PTI, “The vaccine branded as iNCOVACC is likely to be introduced on the Co-WIN platform Friday evening onwards. For now it will be available in private hospitals.” Bharat Biotech’s intranasal COVID vaccine had received approval of the Drugs Controller General of India (DCGI) in November for restricted use in an emergency situation for those above 18 years as a heterologous booster dose. Let’s take a look at this nasal COVID vaccine and why it could be a big deal in the new phase of the battle against the virus. How does it work? According to News18, the vaccine works by using a cold virus from a chimpanzee to deliver a harmless copy of the coronavirus spike protein to the lining of the nose. The concept behind using the intranasal route is that both influenza and COVID-19 follow the same pattern of infection – nose and mouth route into the lungs – which is why mucosal immunity is important. iNCOVACC was developed in partnership with Washington University, St Louis, which had designed and developed the recombinant adenoviral vectored construct and evaluated in pre-clinical studies for efficacy. A press release from the vaccine maker said iNCOVACC, a recombinant replication deficient adenovirus vectored vaccine with a pre-fusion stabilised SARS-CoV-2 spike protein, is the world’s first intranasal vaccine to receive both primary series and heterologous booster approval. The vaccine candidate was evaluated in Phases I, II and III clinical trials with successful results and has been specifically formulated to allow intranasal delivery through nasal drops, it said. The heterologous booster dose studies were conducted for safety and immunogenicity in around 875 subjects, with BBV154 intranasal vaccine administered post two doses of the two commonly administered COVID-19 vaccines, as per Mint. These trials were conducted across nine sites in India. “Immunogenicity was evaluated through serum neutralizing antibodies by PRNT assays and serum IgG’s through ELISA’s. To evaluate vaccine, taken through the intranasal route, IgA’s were evaluated by ELISA in serum and saliva. Evaluation was also carried out for ability iNCOVACC®️ to elicit long-term memory T and B cell responses against the ancestral and omicron variants,” the company said, as per Telangana Today. “We are excited by the expansion of the EUA for iNCOVACC®️ as a booster, which enables this intranasal vaccine to be used by many more people, and hopefully curtail transmission,” said Michael S Diamond, MD, PhD, of Washington University in St. Louis, who co-developed the nasal vaccine technology with Washington University colleague David Curiel, MD, PhD. The vaccine had earlier received approval under Restricted Use in Emergency Situation for ages 18 and above for primary two-dose schedule. Phase-III trials were conducted for safety, immunogenicity in approximately 3,100 subjects, at 14 trial sites across India including All India Institute of Medical Sciences, Delhi and Patna (Bihar), Aatman Hospital, Ahmedabad (Gujarat), PGIMS, Rohtak (Haryana), and Prakhar Hospital, Kanpur, and Rana Hospital, Gorakhpur (Uttar Pradesh). What are its advantages? Bharat Biotech says the nasal delivery system has been designed and developed to be cost-effective in low- and middle-income countries.
Bharat Biotech said iNCOVACC®️ is stable at 2-8°C for easy storage and distribution, as per Telangana Today.
The company has established large manufacturing capabilities at multiple sites across India, including Gujarat, Karnataka, Maharashtra and Telangana, with operations pan India. Economic Times quoted the company as saying that iNCOVACC®️ has the double benefit of enabling faster development of variant-specific vaccines and easy nasal delivery that enables mass immunisation to protect from emerging variants of concern. It promises to become an important tool in mass vaccinations during pandemics and endemics. Bharat Biotech earlier said it is easy to administer as it is non-invasive and does not require trained health care workers. It also eliminates needle-associated risks such as injuries and infections, has higher compliance and is scalable as far as manufacturing is concerned. News18 quoted an article published by Gavi the Vaccine Alliance as noting other advantages including the fact that the sprays don’t need to be refrigerated and don’t need to be administered by health professionals. “People would be able to self-administer them at home,” the article says, adding “they are likely to be more popular for the millions of people who don’t like needles”. Bharat Biotech chairman and MD Krishna Ella told PTI, “Despite the lack of demand for COVID vaccines, we continued product development in intranasal vaccines to ensure that we are well-prepared with platform technologies for future infectious diseases… We have also initiated development of variant-specific vaccines for COVID for future preparedness.” Ella was earlier quoted by news agency PTI as saying that any injectable vaccine only protects the lower level (of the body). That’s why people vaccinated with injectable vaccines may still get RT-PCR positive, whereas the nasal jab protects the whole body. Rajesh S Gokhale, secretary, DBT, and chairperson, BIRAC, said, “The DCGI’s approval of Bharat Biotech’s intranasal vaccine iNCOVACC (BBV154) to be used as a heterologous booster dose against currently available COVID-19 vaccines is a moment of great pride for our country. This move will further strengthen our collective fight against the pandemic and broaden vaccine coverage.” With inputs from agencies Read all the Latest News , Trending News , Cricket News , Bollywood News , India News and Entertainment News here. Follow us on Facebook, Twitter and Instagram.


)

)
)
)
)
)
)
)
)



