New York: Glenmark Generics has recalled its version of oral birth control pills manufactured in India for the US market due to a packaging error that landed the pills in the wrong order and could make the pills ineffective, US health authorities said on Monday. If Americans unintentionally get pregnant because they were on faulty birth control pills, can they sue? In most US states, the answer is yes, according to Brian Palmer in Slate’s Explainer column who researched the faulty contraception issue at length after Pfizer recalled nearly 1 million packages of faulty birth control pills less than a month ago. This time, the US Food and Drug Administration said seven lots of generic norgestimate and ethinyl estradiol tablets which were manufactured and packaged by Glenmark Generics Limited India and distributed to pharmacies across the US between September to 30 December, 2011 were recalled. Glenmark Generics has operated its manufacturing plant in India since 2003. “The recall is being implemented because of a packaging error, where select blisters were rotated 180 degrees within the card, reversing the weekly tablet orientation,” Glenmark said in a statement. [caption id=“attachment_227252” align=“alignleft” width=“380” caption=“Glenmark Generics Inc. announced a nationwide recall of seven lots of Norgestimate and Ethinyl Estradiol Tablets due to the possibility of out of sequence tablets. Pic: US FDA”]
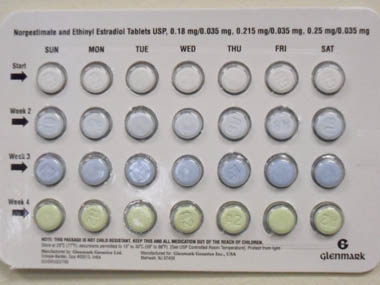 [/caption] “As a result of this packaging error, the daily regimen for these oral contraceptives may be incorrect and could leave women without adequate contraception, and at risk for unintended pregnancy,” it added. The correct packaging aligns 28 tablets in four rows, with the white tablets containing norgestimate and ethinyl estradiol in the top row and light green placebo tablets in the bottom row. Glenmark’s recall will be a matter of concern for any Indian drug exporter. Indian drug makers exporting drugs to America have worked hard to dispel persistent safety concerns. The US Food and Drug Administration (FDA) imposed a temporary ban on more than 30 generic drugs made at Ranbaxy’s Paonta Sahib and Dewas plants in September 2008, alleging manufacturing problems and fraudulent testing. Ranbaxy recently reported its biggest ever quarterly loss after the company took a $500 million charge to settle a legal dispute with US authorities in December. A Pew poll conducted a few years ago showed that 54 percent of Americans distrust drugs made in India and 70 percent distrust drugs from China. “This is extremely disturbing," Steven Goldstein, an obstetrician-gynecologist at New York University Langone Medical Center and Professor at the NYU School of Medicine, told Agency France Press. “This problem with generics manufactured outside of the USA is of great concern to me as a clinician. I often allow and encourage patients to try generics as long as they do not have nuisance side effects however I have always assumed them of equal quality control to the branded products.”
[/caption] “As a result of this packaging error, the daily regimen for these oral contraceptives may be incorrect and could leave women without adequate contraception, and at risk for unintended pregnancy,” it added. The correct packaging aligns 28 tablets in four rows, with the white tablets containing norgestimate and ethinyl estradiol in the top row and light green placebo tablets in the bottom row. Glenmark’s recall will be a matter of concern for any Indian drug exporter. Indian drug makers exporting drugs to America have worked hard to dispel persistent safety concerns. The US Food and Drug Administration (FDA) imposed a temporary ban on more than 30 generic drugs made at Ranbaxy’s Paonta Sahib and Dewas plants in September 2008, alleging manufacturing problems and fraudulent testing. Ranbaxy recently reported its biggest ever quarterly loss after the company took a $500 million charge to settle a legal dispute with US authorities in December. A Pew poll conducted a few years ago showed that 54 percent of Americans distrust drugs made in India and 70 percent distrust drugs from China. “This is extremely disturbing," Steven Goldstein, an obstetrician-gynecologist at New York University Langone Medical Center and Professor at the NYU School of Medicine, told Agency France Press. “This problem with generics manufactured outside of the USA is of great concern to me as a clinician. I often allow and encourage patients to try generics as long as they do not have nuisance side effects however I have always assumed them of equal quality control to the branded products.”
US recalls Glenmark’s India made birth control pills
Uttara Choudhury
• February 28, 2012, 08:11:30 IST
Glenmark’s recall will be a matter of concern for any Indian drug exporter. Indian drug makers exporting drugs to America have worked hard to dispel persistent safety concerns.
Advertisement
)
End of Article