Sun Pharmaceuticals today said said it would buy Ranbaxy Laboratories to create the world’s fifth largest generic company. The deal, which is expected to be completed by the end of 2014, is seen as a harbinger of a wave of consolidation in the Indian pharma sector. It is also one of the major ones that happened in the Indian pharma sector.
Here are five numbers that make the deal stand out. The numbers are taken from the investor presentation of the companies.
STORY CONTINUES BELOW THIS AD
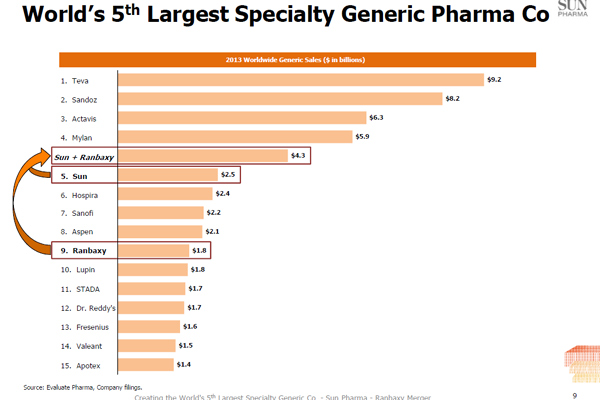
- $4.3 billion: The combined generic sales of Sun Pharma and Ranbaxy. It is the fifth largest number globally. It is still not even half of the world leader Teva. The Israeli company has a sales of $9.2 billion. In the sales sweepstakes, Ranabxy was at the ninth position and Sun Pharma at fifth position.
More from Corporate
[caption id=“attachment_81351” align=“alignleft” width=“600”]
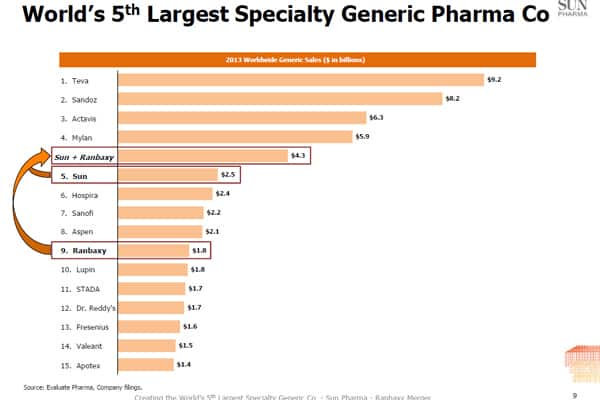 Graph from Sun Ranbaxy Investor presentation.[/caption]
Graph from Sun Ranbaxy Investor presentation.[/caption]
- 9.2%: That is the combined market share of Ranabxy and Sun Pharma in India, much above Abott’s 6.5% which is the second largest post the deal. Prior to the deal, Sun Pharma’s 5.4% market share is the second after Abott and Ranbaxy’s 3.8% is the fifth.
- $1,116 million: The combined sales in India of both the companies as of February. The closest competitor Abbott has just $783 million.
[caption id=“attachment_81352” align=“alignleft” width=“600”]
 Image from Sun Pharma investor presentation.[/caption]
Image from Sun Pharma investor presentation.[/caption]
- $2.2 billion: The combined pro forma revenue from the US as of 31 December. The combined entity will be No. 1 in generic dermatology in the US and No. 3 in branded generics there.
- 184: This is the number of ANDAs the combined entity has filed with the US FDA. This includes high-value first-to-files (FTFs) too. ANDA, or abbreviated new drug application, is essentially a request sumbitted to the US FDA to consider and approve the production and sale of an off-patent drug. FTFs mean the company is the first to submit a request for a generic with complete details.
STORY CONTINUES BELOW THIS AD
End of Article
)